In this post, we discuss a publication in Nature Cell Biology, titled ‘Dapl1 controls NFATc2 activation to regulate CD8+ T cell exhaustion and responses in chronic infection and cancer’ The paper can be accessed here.
As we age, parts of our body become less functional. The same applies at the level of individual immune cells. T cells in our body are less likely to react to foreign antigens as we become older. This phenomenon is called exhaustion.
Exhaustion isn’t just an unfortunate outcome of ageing. It is a regulatory mechanism to curb an uncontrollable immune response, which can cause a cytokine storm (like COVID-19). A prolonged exposure to a specific antigen causes a chronic immune response. In such cases, T cells become exhausted and less functional. In effect, the body is protected from being attacked by its own T cells.
Cancer is one source of foreign antigen that can chronically activate our immune cells. Instead, however, immune activity against cancer is dampened via exhaustion (amongst other mechanisms).
We can give strong immune system back to old people and kill cancer, if we can understand the mechanisms of exhaustion, and how to revert it.
The scientists have focused the function of Dapl1 in T cell exhaustion.
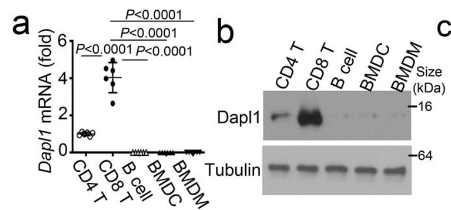
Isolated CD8 T cells express the highest level of Dapl1 compared to other immune cells. The authors show this by performing qPCR and western blot of isolated immune cells.
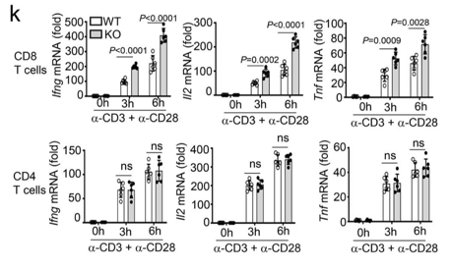
Only CD8 T cells are affected by the deficiency of Dapl1. The authors show this by stimulating CD8 and CD4 T cells with activating antibodies against CD3 and CD28. The expression of effector molecules, such as Ifng, Il2 and Tnf increases only in CD8 T cells.
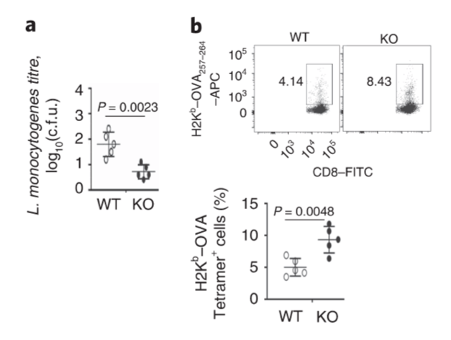
Dapl1-KO (KO) mice have robust response to bacterial infection. The authors infected mice with bacteria called L. monocytogenes. The particular bacterial strain expresses the antigen, called ovalbumin (OVA). KO mice have higher level of OVA-specific CD8 T cells.
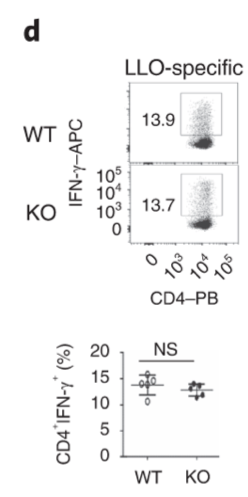
Effector function of CD4 T cells are not affected by Dapl1 deficiency. The percentage of CD4 T cells producing effector molecules are unaffected in KO mice.
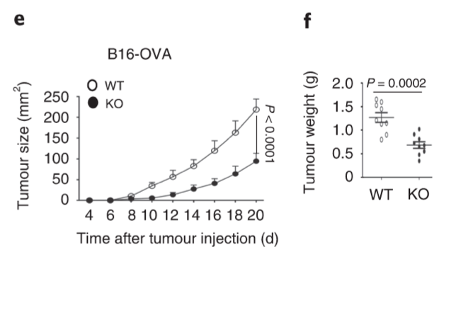
Tumour grows slowly in Dapl1-KO mice. The authors show this by injecting B16-OVA melanoma cell line and monitoring the volume of tumour, and weight of the tumour at the end-point on day 20 after injection.
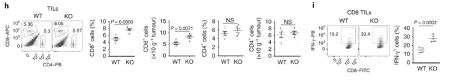
More effector CD8 T cells infiltrate the tumour in Dapl1-KO mice. Dapl1 deficiency causes higher number of CD8+ T cell infiltration in the tumours. The infiltrating CD8+ T cells also produce more pro-inflammatory cytokine, interferon-gamma. The authors show this by profiling the percentage of CD8 T cells and CD4 T cells, and the expression of cytokine, IFN-gamma, by flow cytometry.
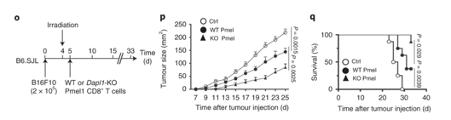
Growth of other tumours are also slowed down in Dapl1-KO mice. The authors use a model of melanoma cell injection, followed by irradiation to deplete the mouse of immune cells. Following irradiation, the mice are given either Dapl1-KO or WT Pmel1-specific CD8+ T cells that recognise and attack melanoma cells. Tumour growth is slowed down in mice that received Dapl1-KO CD8+ T cells.
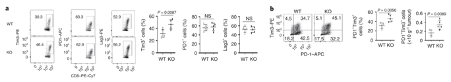
More CD8 T cells express the exhaustion marker, Tim3. Tumour-infiltrating CD8+ T cells in Dapl1 KO mice have higher expression of exhaustion marker Tim3. Figure b is redundant, because what it represents is just a change in percentage of Tim3 expression. The authors the different markers of exhaustion, Tim3, PD1, and Lag3 in KO and WT mice.
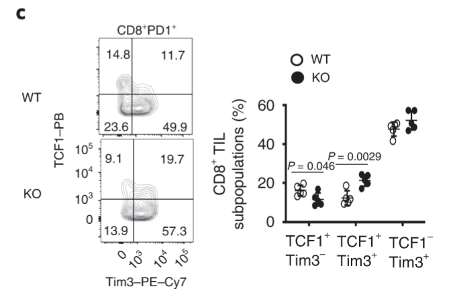
Tim3-expressing CD8 T cells also express higher level of TCF1. TCF1 is a transcription factor that helps to preserve effector functions in exhausted T cells. We see here that the exhausted tumour infiltrating Tim3+ T cells have higher expression of TCF1 in Dapl1 KO mice. Together, the figure suggests that Dapl1 restrains TCF1 to reinforce exhaustion.
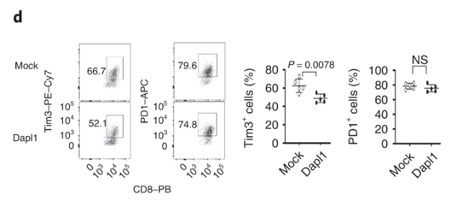
Dapl1 functions intrinsically to CD8 T cells, and it suppresses Tim3 expression. The authors transduce Dapl1 expression in CD8 T cells obtained from Dapl1-KO mice. Transduction of Dapl1 decreases Tim3 expression.
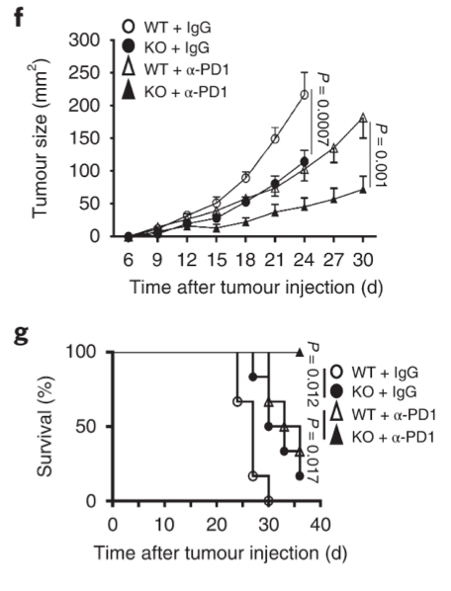
Growth of tumour is strongly inhibited in Dapl1-KO mice receiving PD1 blockade therapy. The authors administer PD1 blocking antibody to tumour-injected mice and monitor their growth. Tumour growth was most strongly inhibited in Dapl1-KO mice receiving PD1 therapy.
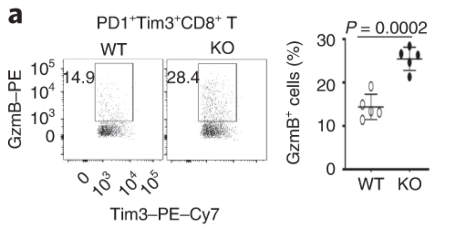
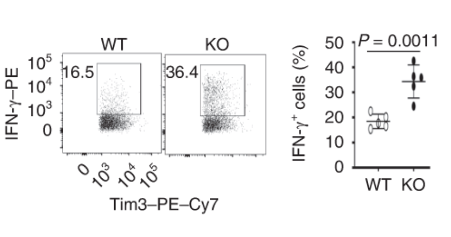
Exhausted CD8 T cells lacking Dapl1 produce more effector molecules than their WT counterpart. Interestingly, Dapl1 KO CD8 T cells express more granzyme B and interferon-gamma, suggesting that they are more functional or effector-like. This observation is in contrast to the fact that Dapl1 deficiency causes increased expression of exhaustion marker, Tim3. The authors profiled the percentage expression of Granzyme B (GzmB) and interferon gamma in tumour infiltrating exhausted T cells.
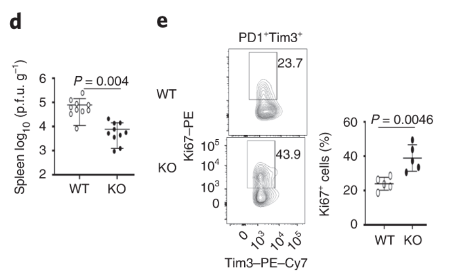
Dapl1-KO CD8 T cells proliferate rapidly in response to viral infection. Dapl1 KO mice have reduced LCMV virus load in the spleen. The CD8 T cells are more proliferative as indicated by the increase percentage of Ki67+ cells.
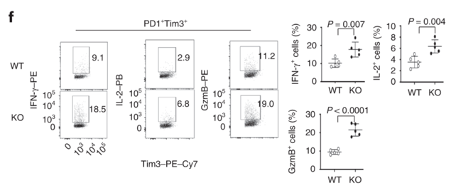
Dapl1-KO CD8 T cells produce more effector molecules in response to viral infection. The authors profiled the percentage expression of effector molecules IFNg, GzmB and IL-2.
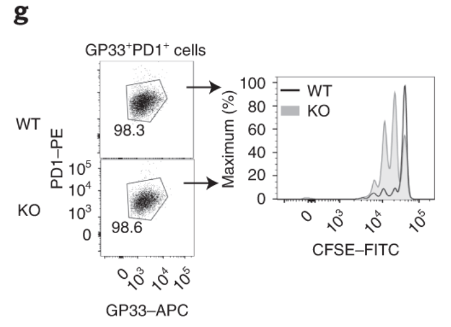
Virus-specific and DApl1-KO GP33+ CD8 T cells proliferate rapidly. The authors cultured virus-specific exhausted CD8 T cells and performed CFSE dilution assay. Note that the CFSE dye is diluted more in KO CD8 T cells.
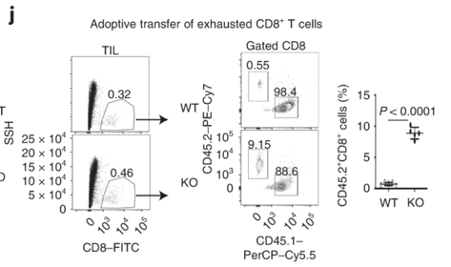
Dapl1-KO CD8 T cells colonise the recipient more readily than the WT CD8 T cells. Interestingly, Tim3+ PD1+ exhausted Dapl1 KO CD8 T cells colonise the recipient mice far better than the exhausted WT CD8 T cells. This suggests that deficiency of Dapl1 or tampering with its function can re-instate effector and proliferative property to the CD8 T cells.
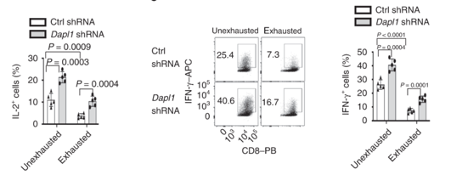
Dapl1 suppresses effector function. As postulated above, knockdown of Dapl1 can increase expression of effector molecule IL-2 and Interferon-gamma in both un-exhausted and exhausted CD8 T cells. The authors have used short hairpin RNA (shRNA) to knockdown Dapl1 expression.
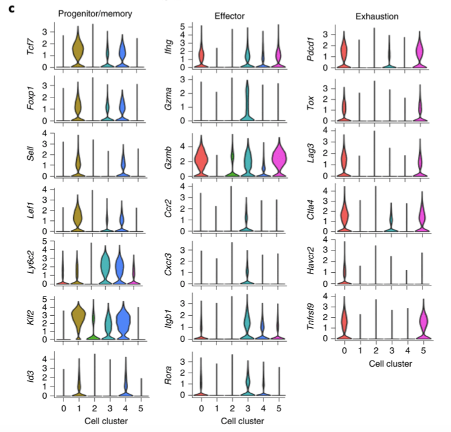
Single cell RNA-seq analysis shows diverse phenotypes of CD8 T cells. The authors performed scRNA-seq of tumour-infiltrating Dapl1 KO and WT CD8 T cells. Analysis generated 5 clusters. Cluster 1 and 4 are associated with progenitor/memory status. Cluster 0 and 5 are associated with exhaustion. Cluster 3 seems to be effector-associated.
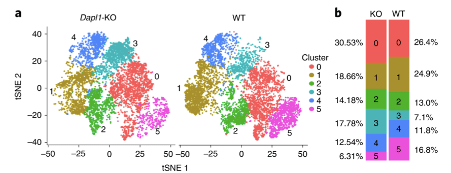
More effector-like CD8 T cells are present amongst Dapl1-KO CD8 T cells than amongst WT CD8 T cells. Notably, the percentage of exhaustion-associated cluster 5 is reduced in Dapl1 KO CD8 T cells. Instead, the effector-associated cluster 3 has drastically increased in percentage. Together, the analysis suggests that there is a reduction in exhaustion and increase in effector sub-populations amongst Dapl1 KO CD8 T cells.
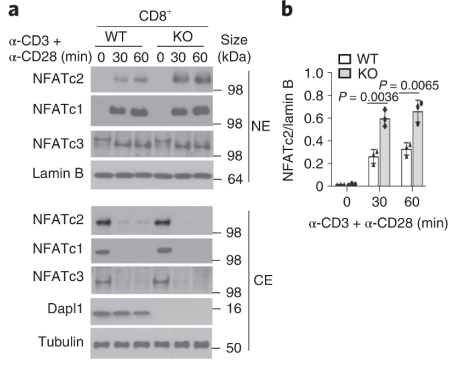
Dapl1 suppresses NFATc2 accumulation in the nucleus. Stimulation of CD3 and CD28 causes more NFATc2 translocation in the Dapl1-KO CD8 T cells.
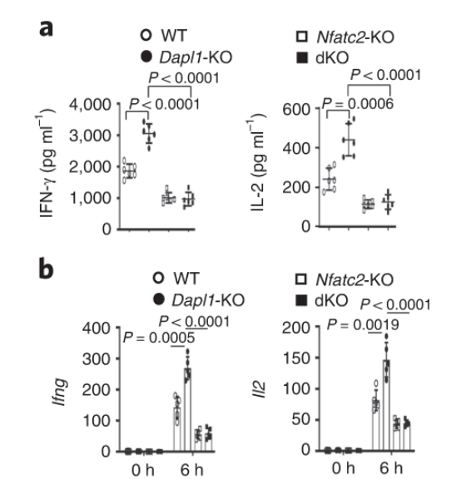
Nfatc2 functions downstream of Dapl1. Expression of effector molecule is completely abolished in Nfatc2-KO CD8 T cells. Taken together with the immunoblot, evidence suggests that Nfatc2 works downstream of Dapl1 to increase expression of effector molecules IL-2 and IFNg.
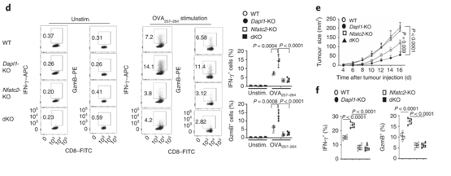
Nfatc2-KO mice are unable to restrain tumour growth. Nfatc2-KO mice have reduced expression of effector molecules. Tumour growth is also unhindered in these mice.
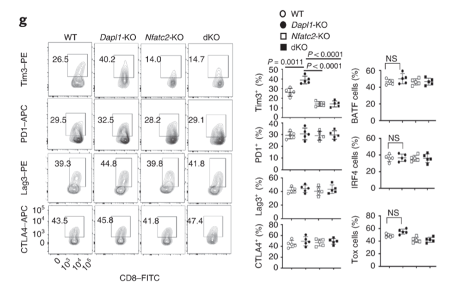
Expression of exhaustion marker Tim3 returns to a basal level in dKO mice.
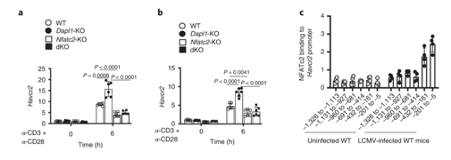
NFATc2 binding to Hacvr2 (Tim3) promoter occurs in response to LCMV infection. The gene encoding Tim3 is called Havcr2. NFATC2 binding to Havcr2 promoter increases in LCMV-infected mice.
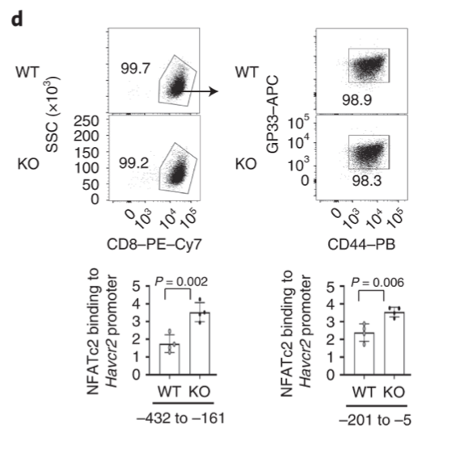
Dapl1 suppresses NFATc2 binding to the Havcr2 promoter. Dapl1 KO mice have increased NFATc2 binding to Havcr2 promoter. This suggests that Dapl1 restrains NFATC2 binding to Havcr2 promoter.
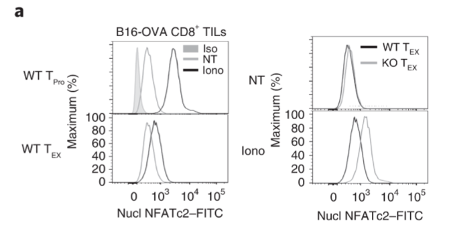
Exhausted CD8 T cells have reduced level of nuclear NFATc2.
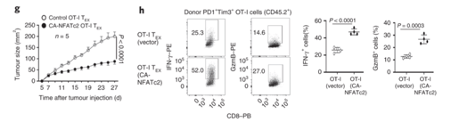
NFATc2 activity is sufficient to restrain tumour growth. Authors transduced exhausted CD8 T cells with constitutively active NFATc2. This resulted in increased expression of effector molecules, and the ability to restrain tumour growth.